
CAR T-cell Therapy Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 180+ Companies Working in the Domain
CAR T-cell therapy is revolutionizing cancer treatment, turning a patient’s own immune cells into precision-guided warriors against tumors. By engineering T-cells with chimeric antigen receptors (CARs), this breakthrough therapy unleashes an army that hunts and destroys cancer with unmatched accuracy. Already delivering life-saving results in blood cancers, CAR T-cell therapy is now pushing boundaries into solid tumors and autoimmune diseases.
/EIN News/ -- New York, USA, March 05, 2025 (GLOBE NEWSWIRE) -- CAR T-cell Therapy Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 180+ Companies Working in the Domain
CAR T-cell therapy is revolutionizing cancer treatment, turning a patient’s own immune cells into precision-guided warriors against tumors. By engineering T-cells with chimeric antigen receptors (CARs), this breakthrough therapy unleashes an army that hunts and destroys cancer with unmatched accuracy. Already delivering life-saving results in blood cancers, CAR T-cell therapy is now pushing boundaries into solid tumors and autoimmune diseases.
DelveInsight’s 'CAR T-cell Therapy Pipeline Insight 2025' report provides comprehensive global coverage of pipeline CAR T-cell therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the CAR T-cell therapy pipeline domain.
Key Takeaways from the CAR T-cell Therapy Pipeline Report
- DelveInsight’s CAR T-cell therapy pipeline report depicts a robust space with 180+ active players working to develop 200+ pipeline CAR T-cell therapies.
- Key CAR T-cell therapy companies such as Cartesian Therapeutics, Arcellx, Inc, Nexcella, Inc, Autolus Therapeutics, Sana Biotechnology, Orgenesis, CARsgen, TILT Biotherapeutics, Poseida Therapeutics, Precision BioSciences, Novartis, Kyverna Therapeutics, Hrain Biotechnology, UTC Therapeutics, Kiromic, Suzhou Fundamenta Therapeutics, Gracell Biotechnology, Innovent Biologics, Nanjing IASO Biotherapeutics, CellabMED, Umoja Biopharma, TC BioPharm, ElevateBio, Century Therapeutics, Allogene Therapeutics, and others are evaluating new CAR T-cell therapies to improve the treatment landscape.
- Promising pipeline CAR T-cell therapies such as Descartes-08, CART-ddBCMA, NXC-201, AUTO-8, SG299, ORGCAR 19.22, KJ-C2113, TILT-123, P-MUC1C-ALLO1, PBCAR-0191, CTL 119, KYV 101, HR 001, Autologous chimeric antigen receptor T-cell therapy, Gamma delta chimeric antigen receptor T-cell therapy, ThisCART 19 A, AZD 0120, CT 071, Equecabtagene autoleucel, CLM 202, UB VV310, CAR-T cell therapy, Research programme: CAR-T cell therapies, CNTY 108, and others are under different phases of CAR T-cell therapy clinical trials.
- In February 2025, Allogene Therapeutics, Inc. announced its participation in an investor conference scheduled for March.
- In February 2025, CARsgen Therapeutics Holdings Limited formed a strategic alliance with Zhuhai Hengqin SB Xinchuang Equity Investment Management Enterprise to fast-track the advancement of allogeneic CAR-T cell products in mainland China.
- In February 2025, Anixa Biosciences, Inc. announced the completion of dosing for the final patient in the third cohort of its ongoing Phase 1 clinical trial, which is assessing its innovative CAR-T cell therapy for recurrent ovarian cancer.
- In February 2025, Hemogenyx Pharmaceuticals reported the first-ever administration of HG-CT-1, its proprietary CAR-T cell therapy, in a human patient. This therapy is designed for adults with relapsed or refractory acute myeloid leukemia (R/R AML). The milestone represents a major step forward in their clinical development, underscoring progress toward offering a potentially life-saving treatment for AML patients with limited alternatives.
- In February 2025, NICE approved CAR T-cell therapy for adults with large B-cell lymphoma, offering significant benefits in delaying disease progression and enhancing survival. Lisocabtagene maraleucel (liso-cel; Breyanzi) is recommended for patients whose cancer is refractory or has relapsed within 12 months of initial chemoimmunotherapy.
Request a sample and discover the recent advances in CAR T-cell therapies @ CAR T-cell Therapy Pipeline Report
The CAR T-cell therapy pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage CAR T-cell therapy, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the CAR T-cell therapy clinical trial landscape.
CAR T-cell Therapy Overview
CAR T-cell therapy is a groundbreaking form of immunotherapy that harnesses a patient's own T cells to fight cancer. The process involves extracting T cells from the patient, genetically modifying them to express a chimeric antigen receptor (CAR) that targets specific cancer cell markers, and then reinfusing the engineered cells back into the body. Once inside, these modified T cells recognize and attack cancer cells with heightened precision. CAR T-cell therapies have demonstrated remarkable success in treating hematologic malignancies such as B-cell leukemias, lymphomas, and multiple myeloma, offering hope to patients who have exhausted conventional treatment options.
Despite its success, CAR T-cell therapy faces several challenges, including high costs, complex manufacturing, and potential side effects such as cytokine release syndrome (CRS) and neurotoxicity. Additionally, its effectiveness in solid tumors remains limited due to issues like the immunosuppressive tumor microenvironment and antigen heterogeneity. Researchers are actively working on next-generation CAR T-cell therapies with improved targeting mechanisms, enhanced persistence, and reduced toxicity. Advances such as allogeneic ("off-the-shelf") CAR T cells and armored CAR T cells are being explored to increase accessibility and effectiveness. As research progresses, CAR T-cell therapy continues to evolve, promising new possibilities for personalized and durable cancer treatment.
Find out more about CAR T-cell therapies @ CAR T-cell Therapy Analysis
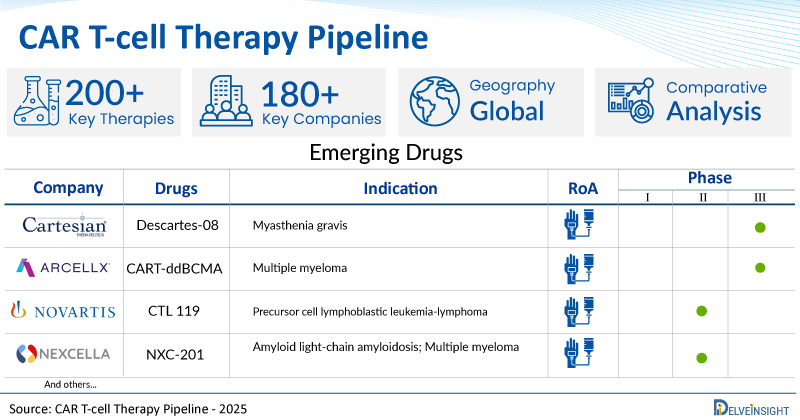
A snapshot of the Pipeline CAR T-cell Therapies mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| Descartes-08 | Cartesian Therapeutics | III | Myasthenia gravis | IV infusion |
| CART-ddBCMA | Arcellx, Inc | III | Multiple myeloma | IV infusion |
| CTL 119 | Novartis | II | Precursor cell lymphoblastic leukemia-lymphoma | IV infusion |
| NXC-201 | Nexcella, Inc | I/II | Amyloid light-chain amyloidosis; Multiple myeloma | IV infusion |
| Azercabtagene zapreleucel | Precision BioSciences | I/II | Non-Hodgkin's lymphoma; Precursor B-cell lymphoblastic leukemia-lymphoma | IV infusion |
| AUTO-8 | Autolus Therapeutics | I | Multiple myeloma | IV infusion |
| Igrelimogene litadenorepvec | TILT Biotherapeutics | I | Malignant melanoma; Non-small cell lung cancer; Ovarian cancer; Solid tumors | IV infusion |
| SG299 | Sana Biotechnology | Preclinical | Chronic lymphocytic leukemia; Non-Hodgkin's lymphoma; Precursor B-cell lymphoblastic leukemia-lymphoma | IV infusion |
| ORGCAR 19.22 | Orgenesis | Preclinical | Precursor B-cell lymphoblastic leukemia-lymphoma | IV infusion |
Learn more about the emerging CAR T-cell therapies @ CAR T-cell Therapy Clinical Trials
CAR T-cell Therapy Therapeutics Assessment
The CAR T-cell therapy pipeline report proffers an integral view of the emerging CAR T-cell therapy segmented by stage, product type, molecule type, and route of administration.
Scope of the CAR T-cell Therapy Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Key CAR T-cell Therapy Companies: Cartesian Therapeutics, Arcellx, Inc, Nexcella, Inc, Autolus Therapeutics, Sana Biotechnology, Orgenesis, CARsgen, TILT Biotherapeutics, Poseida Therapeutics, Precision BioSciences, Novartis, Kyverna Therapeutics, Hrain Biotechnology, UTC Therapeutics, Kiromic, Suzhou Fundamenta Therapeutics, Gracell Biotechnology, Innovent Biologics, Nanjing IASO Biotherapeutics, CellabMED, Umoja Biopharma, TC BioPharm, ElevateBio, Century Therapeutics, Allogene Therapeutics, and others
- Key Pipeline CAR T-cell Therapies: Descartes-08, CART-ddBCMA, NXC-201, AUTO-8, SG299, ORGCAR 19.22, KJ-C2113, TILT-123, P-MUC1C-ALLO1, PBCAR-0191, CTL 119, KYV 101, HR 001, Autologous chimeric antigen receptor T-cell therapy, Gamma delta chimeric antigen receptor T-cell therapy, ThisCART 19 A, AZD 0120, CT 071, Equecabtagene autoleucel, CLM 202, UB VV310, CAR-T cell therapy, Research programme: CAR-T cell therapies, CNTY 108, and others
Dive deep into rich insights for new CAR T-cell therapies, visit @ CAR T-cell Therapies
Table of Contents
| 1. | CAR T-cell Therapy Pipeline Report Introduction |
| 2. | CAR T-cell Therapy Pipeline Report Executive Summary |
| 3. | CAR T-cell Therapy Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | CAR T-cell Therapy Clinical Trial Therapeutics |
| 6. | CAR T-cell Therapy Pipeline: Late-Stage Products (Pre-registration) |
| 7. | CAR T-cell Therapy Pipeline: Late-Stage Products (Phase III) |
| 8. | CAR T-cell Therapy Pipeline: Mid-Stage Products (Phase II) |
| 9. | CAR T-cell Therapy Pipeline: Early-Stage Products (Phase I) |
| 10. | CAR T-cell Therapy Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the CAR T-cell Therapy Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the CAR T-cell Therapy Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the CAR T-cell therapy pipeline therapeutics, reach out @ CAR T-cell Therapy Therapeutics
Related Reports
CAR T-Cell Therapy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CAR T-cell therapy companies, including CytoAgents, Genentech, Incyte Corporation, Caribou Biosciences, Chimeric Therapeutics, Allogene Therapeutics, Kyverna Therapeutics, iCell Gene Therapeutics, Synthekine, Janssen Research & Development, SecuraBio, ImmPACT Bio, A2 Biotherapeutics, Gracell Biopharmaceuticals, Wugen, Sana Biotechnology, Oncternal Therapeutics, Vor Biopharma, CARsgen Therapeutics, Autolus Limited, Arcellx, Kite, Novartis, Lyell Immunopharma, ImmPACT Bio, Tmunity Therapeutics, Poseida Therapeutics, Precigen, AffyImmune Therapeutics, 2seventy bio, Takeda, Cartesian Therapeutics, Cabaletta Bio, Legend Biotech, Miltenyi Biomedicine, Beam Therapeutics, among others.
CAR T-Cell Therapy Competitive Landscape
CAR T-cell Therapy Competitive Landscape – 2025 report provides comprehensive insights about the pipeline and current landscape, pipeline and marketed drug profiles, and the key CAR T-cell therapy companies, including Alnylam Pharmaceuticals, JW Therapeutics, Gilead Sciences, Janssen Pharmaceuticals, Innovent Biologics, Sorrento Therapeutics, Cartesian Therapeutics, CASI Pharmaceuticals, Juventas Cell Therapy, Novartis, Poseida Therapeutics,Shanghai Unicar-Therapy Bio-medicine Technology, Sinobioway Cell Therapy, Tessa Therapeutics, Wuhan Bio-Raid Biotechnology, Miltenyi Biomedicine, Bristol-Myers Squibb, Autolus Limited, Beijing Immunochina Medical Science and Technology, Carsgen Therapeutics, Cellular Biomedicine Group, Chongqing Precision Biotech, Eureka Therapeutics, Formula Pharmaceuticals, Guangzhou Bio-gene Technology, Hebei Senlang Biotechnology, Mustang Bio, MolMed, Aurora BioPharma, Atara Biotherapeutics, Autolus, Bellicum Pharmaceuticals, Kecellitics Biotech Company, Yake Biotechnology, Minerva Biotechnologies, Allogene Therapeutics, PersonGen BioTherapeutics (Suzhou), Precision BioSciences, Pregene (ShenZhen) Biotechnology Company, Shanghai GeneChem, Shanghai Longyao Biotechnology, Shenzhen BinDeBio, among others.
CAR T-Cell Therapy for Non-Hodgkin's Lymphoma Market
CAR T-Cell Therapy for Non-Hodgkin's Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CAR T-Cell therapy for NHL companies, including Allogene Therapeutics, Novartis, Miltenyi Biomedicine, Mustang Bio, CRISPR Therapeutics, 2seventy Bio, Imugene, among others.
CAR T-Cell Therapy for Non-Hodgkin's Lymphoma Pipeline
CAR T-Cell Therapy for Non-Hodgkin's Lymphoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key CAR T-Cell therapy for NHL companies, including Allogene Therapeutics, Novartis, Miltenyi Biomedicine, Mustang Bio, CRISPR Therapeutics, 2seventy Bio, Imugene, among others.
CAR T-Cell Therapy for Multiple Myeloma Market
CAR T-Cell Therapy for Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CAR T-cell therapy for multiple myeloma companies, including Cartesian Therapeutics, Arcellx, Novartis, Bristol-Myers Squibb, CARsgen Therapeutics, Poseida Therapeutics, Juno Therapeutics, CRISPR Therapeutics, bluebird bio, Cellectis SA, Caribou Biosciences, Luminary Therapeutics, Celyad Oncology, Allogene Therapeutics, Gadeta, Nanjing IASO Biotherapeutics, among others.
Oncology Conference Coverage Services
DelveInsight’s Oncology Conference Coverage Services offer a thorough analysis of outcomes from major events like ASCO, ESMO, ASH, AACR, ASTRO, SOHO, SITC, the European CAR T-cell Meeting, and IASLC. This detailed examination provides businesses with essential insights for competitive intelligence and market trend forecasting, supporting the formulation of future strategies.
Get in touch with us today to learn how we can provide AACR coverage exclusively for you at the AACR Meeting 2025
Other Business Consulting Services
Healthcare Competitive Intelligence
Healthcare Portfolio Management
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

