Unlocking a new path to AML treatment: targeting the JMJD1C-RUNX1 axis for leukemia progression control
GA, UNITED STATES, March 3, 2025 /EINPresswire.com/ -- A new study has identified a critical mechanism that could lead to substantial advancements in the treatment of acute myeloid leukemia (AML). Researchers have discovered that the protein JMJD1C plays a pivotal role in leukemia cell survival. Specifically, JMJD1C is recruited by RUNX1 to genomic loci, where it forms liquid-like condensates. This interaction activates key genes essential for the proliferation and survival of AML cells. The findings offer a promising new strategy to target the transcriptional programs driving leukemia, potentially overcoming the disease’s notorious heterogeneity.
Acute myeloid leukemia (AML) is one of the most aggressive and genetically complex cancers, marked by the unchecked growth of immature myeloid cells. Its diversity stems from numerous genetic alterations that disrupt normal blood cell development, presenting significant challenges in developing effective, universal therapies. While current treatments often target specific genetic abnormalities, they fall short of addressing the underlying transcriptional networks that sustain leukemia. Uncovering shared vulnerabilities across AML subtypes has become a pressing priority to devise more inclusive and effective therapeutic strategies.
In a study (DOI: 10.1093/procel/pwae059) published on October 25, 2024, in the journal Protein & Cell, researchers from Tsinghua University and The Rockefeller University revealed an unprecedented role for JMJD1C in regulating gene expression in AML cells. By interacting with RUNX1, a critical transcription factor, JMJD1C drives leukemia cell survival, making it a compelling therapeutic target. This discovery sheds light on how molecular mechanisms underpinning AML could be disrupted to combat this aggressive disease.
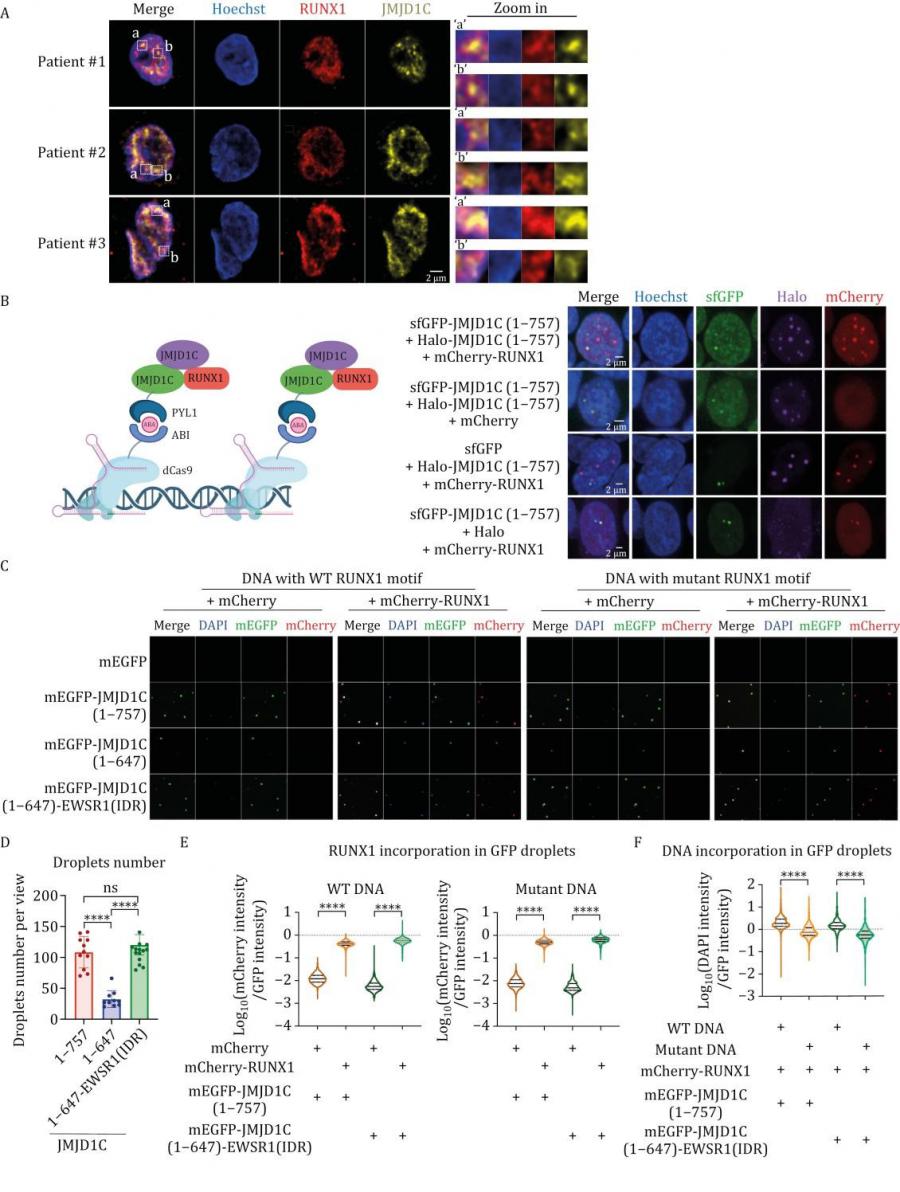
The research delves into how JMJD1C facilitates leukemia cell survival by forming liquid-like condensates through its intrinsically disordered N-terminal region. This unique feature enables JMJD1C to be recruited by RUNX1 to genomic loci, including super-enhancers (SEs). These interactions activate key genes responsible for AML cell proliferation and metabolic processes, maintaining the leukemic state. Importantly, the study highlights that JMJD1C’s non-catalytic functions are critical, with its condensate-forming ability being essential for RUNX1 recruitment and gene regulation. Key experiments revealed that disrupting JMJD1C's N-terminal region impairs its ability to form condensates and interact with RUNX1, leading to reduced leukemia cell viability. Moreover, JMJD1C’s RUNX1-containing condensates might mediate enhancer-promoter interactions crucial for the expression of key leukemic genes regulated by RUNX1. These findings underscore the therapeutic potential of targeting the JMJD1C-RUNX1 axis to halt leukemia progression.
Dr. Mo Chen, one of the senior authors of the study, highlighted the transformative potential of these findings: This research uncovers a previously unappreciated role for JMJD1C in leukemia biology. By elucidating its interaction with RUNX1, we can now envision therapeutic strategies that target this axis across diverse AML subtypes.
The discovery of JMJD1C's role in AML cell survival opens a new frontier in leukemia treatment. By targeting the JMJD1C-RUNX1 interaction, researchers hope to disrupt the transcriptional programs sustaining leukemia cells, offering a universal strategy to tackle AML's heterogeneity. This approach holds promise for overcoming resistance to current therapies and improving patient outcomes. Future research will focus on translating these molecular insights into clinical interventions, heralding a new era in the fight against leukemia.
DOI
10.1093/procel/pwae059
Original Source URL
https://doi.org/10.1093/procel/pwae059
Funding information
This work was funded by National Key R&D Program of China (grant 2021YFA1300100 to M.C.), Beijing Municipal Natural Science Foundation (grant JQ23024 to M. C.), Leukemia and Lymphoma Society (grant 7021-20 to R.G.R), National Natural Science Foundation of China (grant 32300445 to Q.C.) and a Tsinghua-Peking Center for Life Sciences postdoctoral fellowship to Q.C..
Lucy Wang
BioDesign Research
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release