
Motor Neuron Disease Clinical Trial Pipeline Appears Robust With 120+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The market growth rate is significantly impacted by the rising prevalence of motor neuron disease and the increasing geriatric population. Additionally, the burgeoning demand for advanced and efficient treatment options, coupled with a rise in healthcare spending, expanding government funding, and concerted initiatives by public and private organizations to raise awareness about this rare disease and its treatment options, are key factors set to expand the motor neuron disease (MND) market.
/EIN News/ -- New York, USA, May 16, 2024 (GLOBE NEWSWIRE) -- Motor Neuron Disease Clinical Trial Pipeline Appears Robust With 120+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The market growth rate is significantly impacted by the rising prevalence of motor neuron disease and the increasing geriatric population. Additionally, the burgeoning demand for advanced and efficient treatment options, coupled with a rise in healthcare spending, expanding government funding, and concerted initiatives by public and private organizations to raise awareness about this rare disease and its treatment options, are key factors set to expand the motor neuron disease (MND) market.
DelveInsight’s 'Motor Neuron Disease Pipeline Insight 2024' report provides comprehensive global coverage of pipeline motor neuron disease therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the motor neuron disease pipeline domain.
Key Takeaways from the Motor Neuron Disease Pipeline Report
- DelveInsight’s motor neuron disease pipeline report depicts a robust space with 120+ active players working to develop 130+ pipeline therapies for motor neuron disease treatment.
- Key motor neuron disease companies such as Biohaven Pharmaceuticals, Inc., Prilenia Therapeutics, Helixmith Co., Ltd., Transposon Therapeutics, Inc., GeneCradle Therapeutics, Verge Genomics, QurAlis Corporation, Zydus Lifesciences Limited, Ra Pharmaceuticals, Guangzhou Magpie Pharmaceuticals Co., Ltd., Scholar Rock, Inc., Spinogenix, Seelos Therapeutics, Inc., Hoffmann-La Roche, Revalesio Corporation, Rapa Therapeutics LLC, Q Therapeutics, Inc., PTC Therapeutics, ProJenX, NeuroSense Therapeutics Ltd., Knopp Biosciences, MediciNova, Amylyx Pharmaceuticals, NMD Pharma A/S, Supernus Pharmaceuticals, Coya Therapeutics, OrphAI Therapeutics, Ionis Pharmaceuticals, UniQure Biopharma, Annexon, Cellenkos, MaaT Pharma, and others are evaluating new motor neuron disease drugs to improve the treatment landscape.
- Promising motor neuron disease pipeline therapies such as Talditercept alfa, Pridopidine, VM202, Censavudine, GC 101, VRG 50635, QRL 201, ZYIL1, Zilucoplan, Tetramethylpyrazine nitrone, SRK-015, SPG302, SLS-005, RO7204239, RNS60, RAPA-501, Q-Cells, PTC857, Prosetin, PrimeC, Dexpramipexole, MN-166, NMD670, MYOBLOC, COYA 302, AIT-101, ION363, AMT-162, ANX005, CK0803, MaaT033, and others are under different phases of motor neuron disease clinical trials.
- In April 2024, BrainStorm Cell Therapeutics Inc., a leading developer of adult stem cell therapeutics for neurodegenerative diseases, announced that it received written agreement from the US Food and Drug Administration (FDA), under a Special Protocol Assessment (SPA), on the design for a Phase IIIb trial of NurOwn in amyotrophic lateral sclerosis.
- In April 2024, Prilenia Therapeutics announced the presentation of the latest research from the pridopidine Huntington's disease and amyotrophic lateral sclerosis programs at the American Academy of Neurology (AAN) Annual Congress, in Denver, Colorado, April 13-18.
- In March 2024, Seelos Therapeutics, Inc. provided an update on top-line data of the Phase II/III HEALEY ALS Platform trial. This study was performed in collaboration with The Sean M. Healey and AMG Center, which is viewed as an influential force in ALS research and in caring for the ALS community. The study was designed to evaluate SLS-005 (IV trehalose), a low molecular weight disaccharide that stabilizes misfolded proteins and activates autophagy, in decreasing the slope of the ALS Functional Rating Scale (ALSFRS-R) and separation from placebo in Function and Mortality in an all-comers population of Persons with ALS.
- In March 2024, Verge Genomics and Spanish biopharma Ferrer entered a strategic collaboration to co-develop VRG50635, Verge’s lead drug candidate for the treatment of sporadic and familial forms of amyotrophic lateral sclerosis in Europe, Central and South America, Southeast Asia, and Japan.
- In March 2024, OrphAI Therapeutics’ experimental therapy AIT-101 was awarded orphan drug status in the European Union for amyotrophic lateral sclerosis (ALS), following a similar designation granted in the US last year.
- In February 2024, NeuroSense Therapeutics, a company developing treatments for severe neurodegenerative diseases, reported additional positive data from its six-month double-blind Phase IIb PARADIGM trial of NeuroSense's lead drug candidate PrimeC for the treatment of amyotrophic lateral sclerosis.
- In January 2024, Voyager Therapeutics, Inc. announced a strategic collaboration and capsid license agreement with Novartis Pharma AG, a subsidiary of Novartis AG (NYSE: NVS) to advance potential gene therapies for Huntington’s disease (HD) and spinal muscular atrophy (SMA). Voyager will provide Novartis a target-exclusive license to access Voyager’s TRACER™ capsids and other intellectual property for the respective diseases, and Voyager and Novartis will collaborate to advance a preclinical gene therapy candidate for HD.
- In February 2023, Biohaven Ltd. announced that it had received Fast Track designation from the US Food and Drug Administration (FDA) for taldefgrobep alfa, a novel anti-myostatin adnectin, for the treatment of spinal muscular atrophy.
Request a sample and discover the recent advances in motor neuron disease treatment drugs @ Motor Neuron Disease Pipeline Report
The motor neuron disease pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage motor neuron disease drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the motor neuron disease clinical trial landscape.
Motor Neuron Disease Overview
Motor neuron diseases are a collection of progressive neurological conditions that harm motor neurons, the cells responsible for controlling activities like walking, breathing, talking, and swallowing. This category encompasses illnesses such as amyotrophic lateral sclerosis, progressive bulbar palsy, primary lateral sclerosis, progressive muscular atrophy, spinal muscular atrophy, Kennedy's disease, and post-polio syndrome. Motor neuron disease (MND) is an uncommon disorder affecting the brain and nerves, leading to a worsening weakness over time. The signs of this disease emerge slowly and might not be immediately noticeable. Initial symptoms can include difficulty with ankle or leg strength, resulting in tripping or difficulty with stairs; slurred speech, which can progress to trouble swallowing certain foods; weakened grip, leading to dropping objects or struggling with tasks like opening jars or fastening buttons; muscle cramps and twitches; weight loss due to muscle deterioration in the arms or legs; and difficulty controlling laughter or tears in inappropriate situations.
Diagnosing motor neuron diseases often presents challenges as there are no definitive tests in many cases. Motor neuron disease symptoms can vary widely among individuals and, in the early stages, may resemble those of other illnesses, complicating diagnosis. However, specific gene tests are available for conditions like SMA, Kennedy's disease, and some forms of familial ALS. A thorough physical examination followed by an extensive neurological assessment is typically necessary. These combined tests can distinguish between muscle diseases and motor neuron diseases. Electromyography (EMG) is utilized to diagnose lower motor neuron disorders, along with a nerve conduction study.
Treatment options for motor neuron diseases include botulinum toxin injections to alleviate muscle stiffness by weakening overactive muscles. These injections may be administered in the salivary glands to manage drooling. Excessive saliva production can also be addressed with medications such as amitriptyline, glycopyrrolate, and atropine. Physical therapy and rehabilitation programs can aid in improving posture, preventing joint immobility, and slowing muscle weakness and wasting. Stretching and strengthening exercises are beneficial for reducing stiffness, enhancing range of motion, and improving circulation.
Find out more about motor neuron disease treatment drugs @ Drugs for Motor Neuron Disease Treatment
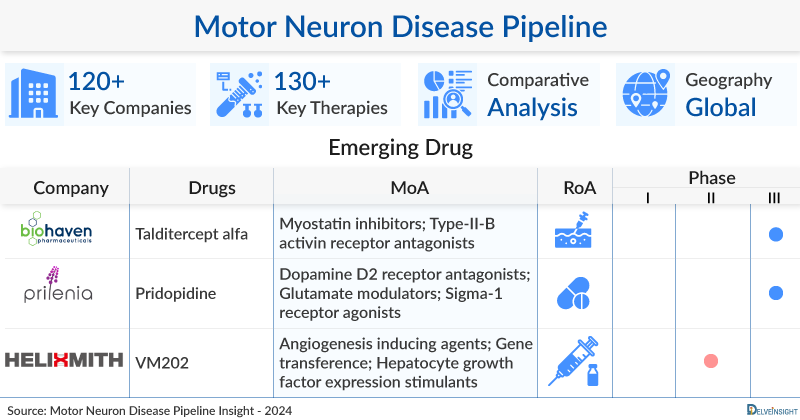
A snapshot of the Motor Neuron Disease Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Talditercept alfa | Biohaven Pharmaceuticals, Inc. | Phase III | Myostatin inhibitors; Type-II-B activin receptor antagonists | Subcutaneous |
| Pridopidine | Prilenia Therapeutics | Phase III | Dopamine D2 receptor antagonists; Glutamate modulators; Sigma-1 receptor agonists | Oral |
| VM202 | Helixmith Co., Ltd. | Phase II | Angiogenesis inducing agents; Gene transference; Hepatocyte growth factor expression stimulants | Intramuscular |
| TPN-101 | Transposon Therapeutics, Inc. | Phase II | Nucleoside reverse transcriptase inhibitors | Oral |
| GC 101 | GeneCradle Therapeutics | Phase I/II | Gene transference | Intrathecal |
| VRG 50635 | Verge Genomics | Phase I | PIKFYVE protein inhibitors | Oral |
| QRL 201 | QurAlis Corporation | Phase I | Stathmin modulators | Intrathecal |
Learn more about the emerging motor neuron disease pipeline therapies @ Motor Neuron Disease Clinical Trials
Motor Neuron Disease Therapeutics Assessment
The motor neuron disease pipeline report proffers an integral view of the motor neuron disease emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Motor Neuron Disease Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravitreal, Subretinal, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Myostatin inhibitors, Type-II-B activin receptor antagonists, Dopamine D2 receptor antagonists, Glutamate modulators, Sigma-1 receptor agonists, Angiogenesis inducing agents, Gene transference, Hepatocyte growth factor expression stimulants, Nucleoside reverse transcriptase inhibitors, PIKFYVE protein inhibitors, Stathmin modulators
- Key Motor Neuron Disease Companies: Biohaven Pharmaceuticals, Inc., Prilenia Therapeutics, Helixmith Co., Ltd., Transposon Therapeutics, Inc., GeneCradle Therapeutics, Verge Genomics, QurAlis Corporation, Zydus Lifesciences Limited, Ra Pharmaceuticals, Guangzhou Magpie Pharmaceuticals Co., Ltd., Scholar Rock, Inc., Spinogenix, Seelos Therapeutics, Inc., Hoffmann-La Roche, Revalesio Corporation, Rapa Therapeutics LLC, Q Therapeutics, Inc., PTC Therapeutics, ProJenX, NeuroSense Therapeutics Ltd., Knopp Biosciences, MediciNova, Amylyx Pharmaceuticals, NMD Pharma A/S, Supernus Pharmaceuticals, Coya Therapeutics, OrphAI Therapeutics, Ionis Pharmaceuticals, UniQure Biopharma, Annexon, Cellenkos, MaaT Pharma, and others
- Key Motor Neuron Disease Pipeline Therapies: Talditercept alfa, Pridopidine, VM202, Censavudine, GC 101, VRG 50635, QRL 201, ZYIL1, Zilucoplan, Tetramethylpyrazine nitrone, SRK-015, SPG302, SLS-005, RO7204239, RNS60, RAPA-501, Q-Cells, PTC857, Prosetin, PrimeC, Dexpramipexole, MN-166, NMD670, MYOBLOC, COYA 302, AIT-101, ION363, AMT-162, ANX005, CK0803, MaaT033, and others
Dive deep into rich insights for new drugs for motor neuron disease treatment, visit @ Motor Neuron Disease Drugs
Table of Contents
| 1. | Motor Neuron Disease Pipeline Report Introduction |
| 2. | Motor Neuron Disease Pipeline Report Executive Summary |
| 3. | Motor Neuron Disease Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Motor Neuron Disease Clinical Trial Therapeutics |
| 6. | Motor Neuron Disease Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Motor Neuron Disease Pipeline: Late-Stage Products (Phase III) |
| 8. | Motor Neuron Disease Pipeline: Mid-Stage Products (Phase II) |
| 9. | Motor Neuron Disease Pipeline: Early-Stage Products (Phase I) |
| 10. | Motor Neuron Disease Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Motor Neuron Disease Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Motor Neuron Disease Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the motor neuron disease pipeline therapeutics, reach out @ Motor Neuron Disease Treatment Drugs
Related Reports
Motor Neuron Disease Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key motor neuron disease companies, including Ra Pharmaceuticals, Guangzhou Magpie Pharmaceuticals Co., Ltd., Scholar Rock, Inc., Spinogenix, Seelos Therapeutics, Inc., Sanofi, Hoffmann-La Roche, Revalesio Corporation, Cytokinetics, Rapa Therapeutics LLC, Q Therapeutics, Inc., PTC Therapeutics, ProJenX, NeuroSense Therapeutics Ltd., Knopp Biosciences, MediciNova, among others.
Motor Neuron Disease Epidemiology
Motor Neuron Disease Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted motor neuron disease epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Amyotrophic Lateral Sclerosis Pipeline
Amyotrophic Lateral Sclerosis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key amyotrophic lateral sclerosis companies, including Biogen, Ionis Pharmaceuticals, Ferrer Internacional S.A., AbbVie, Calico Life Sciences LLC, Genuv Inc., Kadimastem, Corcept Therapeutics, AL-S Pharma, Rapa Therapeutics LLC, Cytokinetics, MediciNova, Retrotope, Inc. Woolsey Pharmaceuticals, Sanofi, PTC Therapeutics, Helixmith Co., Ltd., Annexon, Inc., Denali Therapeutics Inc., Revalesio Corporation, Clene Nanomedicine, Ashvattha Therapeutics, Inc., Apellis Pharmaceuticals, Inc., Procypra Therapeutics, Knopp Biosciences, InFlectis BioScience, AI Therapeutics, Inc., Cellenkos, ZZ Biotech, LLC, QurAlis Corporation, Alector Inc., NeuroSense Therapeutics Ltd., Novartis Pharmaceuticals, Eledon Pharmaceuticals, among others.
Duchenne Muscular Dystrophy Market
Duchenne Muscular Dystrophy Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Duchenne muscular dystrophy companies including Sarepta Therapeutics, PTC Therapeutics, Nippon Shinyaku, Pfizer, Santhera Pharmaceuticals, ReveraGen BioPharma, Taiho Pharmaceutical, FibroGen, Capricor, Daiichi Sankyo, Italfarmaco, Antisense Therapeutics, Solid Biosciences, among others.
Nonsense Mutation Duchenne Muscular Dystrophy Pipeline
Nonsense Mutation Duchenne Muscular Dystrophy Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key nonsense mutation Duchenne muscular dystrophy companies, including Taiho Pharmaceutical, Solid Biosciences, Capricor, Nippon Shinyaku, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

