RCE Technologies, Inc. Announces First-In-Human Transdermal Continuous Cardiac Biomarker Monitoring
Latest Research Results Involving Groundbreaking Wearable Device Unveiled at American College of Cardiology Conference in Atlanta (ACC.24)
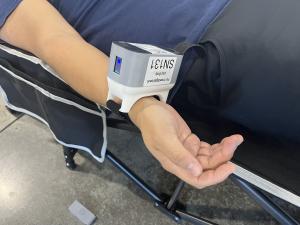
Featured in an ACC.24 “Spotlight on Special Topics” presentation showcasing pioneering advancements in cardiac monitoring technology, the study marks a significant milestone in medical innovation. It was spearheaded by Ronald P. Karlsberg, MD, a Fellow of The American College of Cardiology and clinical professor of Medicine at both Cedars-Sinai Institute and UCLA, and led by Suhail Y. Dohad, MD, as principal investigator. Working with 20 consecutive patients encompassing both scheduled diagnostic catheterization procedures and emergency cases of heart attacks, clinical staff applied the Infrasensor wearable device to patients’ wrists before the procedure, enabling real-time continuous monitoring throughout coronary catheterization, extended monitoring in the post-anesthesia care unit (PACU), and removal just before hospital discharge.
“This is an innovative platform for measuring biomarkers,” stated Dr. Karlsberg. “The insights underscore the significant promise of this novel technology, which inaugurates a new era for monitoring in a wide variety of scenarios real-time and affords the opportunity to develop and refine diagnostic pathways that will have a substantial impact on patient care.”
The study builds on earlier findings presented at the ACC 2023 late-breaking clinical trials, where it was posited that exploring real-time biomarker trends from the wrist wearable could further the understanding of the transdermal signal and the association with clinically relevant use cases at the point of care.
RCE Technologies’ Founder/CEO Atandra Burman commented, “We are honored to have the collective dedication of esteemed clinicians and key opinion leaders in pushing the boundaries of medical exploration. This study highlights the value of digital biomarkers that may enable instantaneous feedback for care providers in assessing the patient. The insights lead us towards refining our understanding of the transdermal signals and our approach towards developing improved heart attack diagnostics.”
About RCE: RCE.ai, an innovative artificial intelligence (AI) based medical technology company launched in Carlsbad, Calif. in 2018, is dedicated to early heart attack detection. The company has revolutionized the monitoring of cardiac injury by introducing, Infrasensor™, a non-invasive infrared-based wearable device. Its proprietary transdermal approach, enhanced with deep learning techniques, analyzes and associates unique optical signatures with cardiac conditions in real-time. This AI coupled transdermal approach enables the potential application of RCE’s technology in various emergency care settings and remote monitoring applications. Through ongoing clinical studies to optimize its product for clinical use, the company hopes to soon secure FDA approval for its technology, opening the door to making it available for sale in the United States. For more information, please visit https://rce.ai/contact-us/, follow us on LinkedIn or X (formerly Twitter), or contact Founder/CEO Atandra Burman at atandra@rce.ai.
GREG PITKOFF
GRiP Communications LLC
+1 718-614-6677
greg@gripcommpr.com
Visit us on social media:
Twitter
LinkedIn
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.