PKG, Inc. becomes key manufacturer for ClearMask™ and Xometry
The first fully transparent, FDA-cleared mask produced in the USA is made possible through a manufacturing partnership between ClearMask LLC, Xometry, and PKG.
Cerebain BioTech Corp. (OTCMKTS:CBBT)
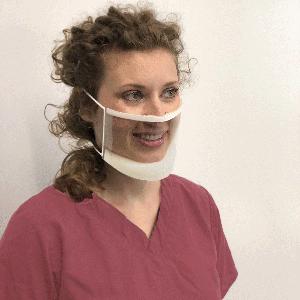
ClearMask™ is the world's first FDA-cleared, fully transparent surgical mask suitable for hospitals, clinics, schools, retail, hospitality, and other settings. The mask is optimized for maximum clarity and comfort, and meets applicable ASTM Level 3 requirements for fluid resistance and flammability, which offers a high level of protection for medical use in environments such as operating rooms.
“In this disheartening time as the world fights against the COVID-19 pandemic, the ClearMask™ helps protect while bringing much-needed relief through a reassuring smile and familiar face among fear and confusion and suffering. In addition to blocking particles or droplets with its fully transparent, anti-fog plastic barrier, the ClearMask™ helps improve visual communication, which may help avoid costly errors and adverse outcomes. Transparent communication during the customer experience can be critical in establishing rapport and earning trust while assuring safety as a priority,” announced ClearMask in its press release.
To meet the rigorous controls necessary for FDA-cleared products, ClearMask LLC and Xometry, the largest marketplace for On-Demand manufacturing, partnered with PKG Inc., an ISO 13485-certified facility, to manufacture the FDA-cleared version of the ClearMask™. PKG will provide valuable experience in building FDA-approved products, enabling mass production while achieving rigorous oversight and high quality.
This November, PKG Inc. officially kick-started a new production floor designed to meet the mass production of the FDA-cleared ClearMask™, which has been in high demand.
Over a few weeks, PKG Inc. will fully transition company ownership to the Cerebain Biotech Corporation in an acquisition that began in the middle of this year. Upon completion, under the Cerebain Biotech umbrella, PKG Inc. will continue its support in mass-producing large volumes of the FDA-cleared ClearMask™. "We are proud to help ClearMask fight against the pandemic with a smile on our face that everyone can see." -- ALMIR GARIBOVIC, PRESIDENT OF PKG
"All of us are looking forward to successfully providing innovative accessibility and protection to diverse populations,” stated PKG CEO Almir Garibovic.
If you would like to order the FDA-cleared ClearMask™, visit: https://buy.theclearmask.com/
About ClearMask™
ClearMask™, LLC is an American-based medical supply company that focuses on improving communication while making connections more human. Its products may help reduce errors and increase satisfaction by facilitating visual communication for all. For more information, please visit www.theclearmask.com
About Xometry
Xometry is the largest marketplace for custom manufacturing, connecting customers with optimal manufacturing solutions through proprietary AI algorithms. Xometry provides on-demand manufacturing and industrial supply materials to a diverse customer base, ranging from startups to Fortune 100 companies, including BMW, Dell Technologies, General Electric, Bosch, and NASA. Our nationwide network of thousands of supplier manufacturing facilities enables us to maintain consistently fast lead times while offering a broad array of capabilities, including CNC machining, 3D printing, sheet metal fabrication, injection molding, and urethane casting. Additionally, Xometry offers its supplier network an array of financing solutions to help improve cash flow and achieve better growth and efficiency.
Learn more about Xometry at www.xometry.com or on Twitter at @xometry.
About PKG
PKG, Inc. specializes in contract design, development, and manufacturing of system-level devices with expertise in human-machine interfaces. With experience in medical, aerospace, government, and industrial products, PKG helps raise your company to the next level by leveraging our engineering and technology integration skills across your product lines. Complete in-house vertical integration of expertise, services, manufacturing processes, and technologies, PKG provides customers with all their product development and manufacturing needs. Besides expertise in system-level devices and human-machine interfaces, PKG offers incubation and acceleration services for startup businesses and entrepreneurs. For more information, visit us at www.pkguis.com
About Cerebain Biotech Corp.
Cerebain Biotech Corp. (OTC: CBBT) is a development-stage medical device company focused on the creation and clinical development of a minimally invasive implantable device and a synthetic drug solution. The device leverages the clinically observable, positive impact that Omentum stimulation has on cognitive function as related to dementias, and in particular, Alzheimer’s disease. The corporate vision is based on these positive clinical observations. Visit us at www.cerebain.com or connect with us on Twitter and Facebook to learn more.
Jonathan Frank
PKG, Inc.
+1 208-577-5160
jfrank@pkguis.com
Visit us on social media:
Facebook
Twitter
LinkedIn
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.